A Roadmap for Safe and Transparent Use of AI in Medically Assisted Reproduction (MAR)
Presented at ESHRE 2025 by Dr Cristina Hickman and the AI Fertility Society
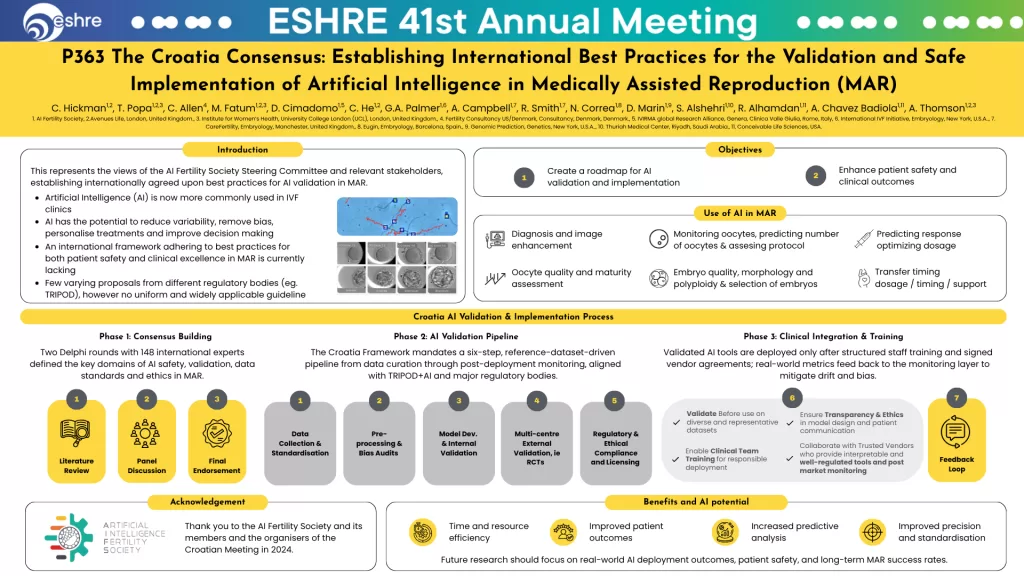
Why We Need a Global Framework for AI Validation in Reproductive Medicine
Artificial Intelligence is increasingly used in IVF — from embryo selection to protocol optimisation — but adoption is far ahead of regulation.
Many AI tools enter clinical workflows without clear validation standards, shared safety protocols, or consistent reporting structures. That gap leaves clinics, developers, and patients at risk.
The Croatia Consensus, led by the AI Fertility Society, brings together global experts across reproductive medicine, embryology, AI development, and bioethics to address this.
What Is the Croatia Consensus?
The Croatia Consensus is the first international framework for the validation and safe implementation of AI in medically assisted reproduction (MAR).
It outlines:
- A clear pipeline for validation — from dataset curation to post-deployment monitoring
- Safety and ethics protocols
- Integration of widely recognised reporting standards like TRIPOD+AI
- Steps for training, transparency, and vendor accountability
The 3 Phases of AI Validation in MAR
Phase 1: Consensus Building
- Two Delphi rounds with 148 experts
- Defined key domains for AI validation: safety, data standards, bias mitigation, and clinical integration
Phase 2: AI Validation Pipeline
A six-step process:
- Data collection and standardisation
- Preprocessing and bias audits
- Model development and internal validation
- Multi-centre external validation (including RCTs)
- Regulatory and ethical compliance
- Transparency in model design and patient communication
Phase 3: Clinical Integration and Training
- Tools deployed only after structured staff training
- Real-world feedback loops to detect model drift
- Collaboration with vendors who support post-market monitoring
Why This Matters: Moving from Innovation to Safe Implementation
The use of AI in MAR is growing rapidly, but the lack of harmonised global guidance introduces risk:
- Tools validated only on narrow datasets may underperform in diverse populations
- Models lacking explainability may undermine clinician trust
- Without structured oversight, model drift and bias can silently degrade outcomes
The Croatia Consensus addresses these risks and offers a practical, scalable framework for IVF clinics, researchers, regulators, and AI developers.
Key Outcomes and Benefits
By adopting the framework outlined in the Croatia Consensus, stakeholders can:
- Improve patient safety and clinical outcomes
- Enhance predictive performance of AI tools
- Reduce operational and ethical risks
- Ensure that AI models remain effective, interpretable, and aligned with evolving care standards
Limitations and Future Focus
The framework is expert-driven and informed by current scientific literature — but like AI itself, it must evolve.
Next steps include:
- Testing the framework in real-world clinical settings
- Monitoring long-term patient outcomes
- Expanding alignment with global regulatory authorities
Conclusion: A Blueprint for Responsible AI in Fertility Care
The Croatia Consensus provides what’s been missing — a validated, ethically grounded, and clinically focused roadmap for safe AI adoption in IVF.
By building consensus across disciplines and borders, it offers a clear path forward for AI innovation that earns trust, improves care, and puts patients first.