Linking Oocyte Quality to Embryo Viability with AI
The Question: Can a Good Egg Predict a Good Embryo?
One of the most debated questions in embryology is whether oocyte quality determines the fate of the embryo.
Until now, this question has mostly been answered through experience, morphology, and subjective judgment. But AI is offering a new lens.
At Avenues, our team partnered with Fairtility to evaluate whether AI-generated oocyte quality (OQ) scores can reliably predict:
- Embryo quality (EQ)
- Fertilisation type (normal or abnormal)
This study is one of the first large-scale efforts to examine the oocyte-to-embryo relationship using objective, AI-driven metrics rather than manual assessment alone.
What Is CHLOE OQ and Why Does It Matter?
CHLOE OQ uses a single time-lapse image to generate an oocyte quality score based on AI-analyzed features.
CHLOE EQ evaluates embryos across all time-lapse frames to generate an embryo quality score.
These tools offer a more standardized, data-driven approach to grading gametes and embryos — and the question we asked was simple: can a single image of an egg help predict which embryos will succeed?
Study Summary: Connecting OQ and EQ
Design and Methodology
- Retrospective cohort study
- 1,240 embryos from IVF/ICSI cycles between January 2024 and January 2025
- Single-centre study at Avenues
- OQ and EQ scores generated via CHLOE OQ and CHLOE EQ
- Statistical analysis performed using ANOVA and Tukey’s HSD
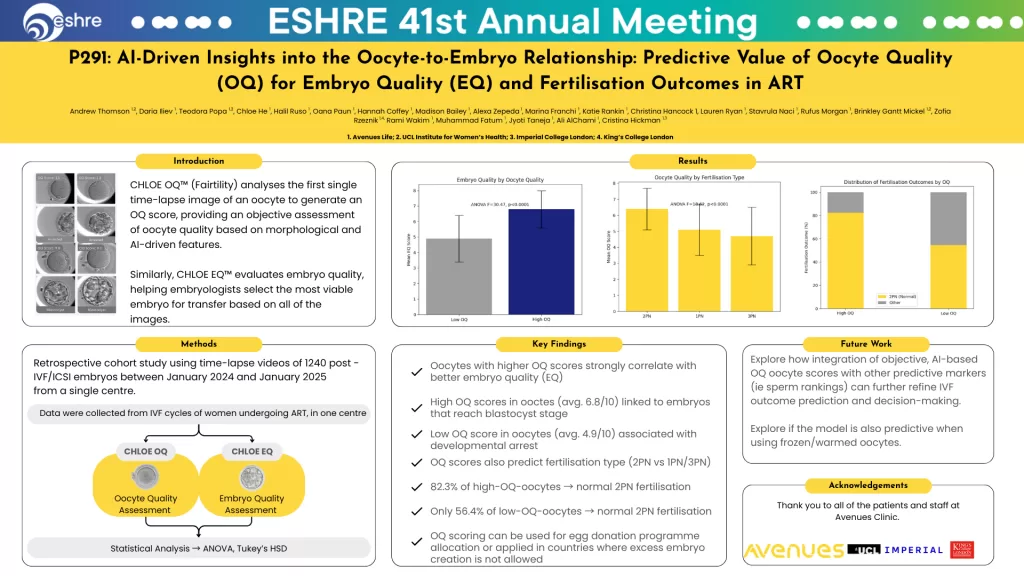
Key Findings: OQ Scores Predict Embryo Quality and Fertilisation Outcomes
Finding 1: Higher OQ = Higher EQ
- Oocytes with high OQ scores (average 6.8/10) were significantly more likely to reach the blastocyst stage
- Oocytes with low OQ scores (average 4.9/10) were associated with developmental arrest
- High OQ oocytes produced embryos with better morphology and faster cleavage rates
Finding 2: OQ Predicts Fertilisation Type
- 82.3 percent of high-OQ oocytes resulted in normal 2PN fertilisation
- Only 54.6 percent of low-OQ oocytes resulted in normal 2PN fertilisation
- OQ scores for abnormally fertilised oocytes (1PN, 3PN) were significantly lower
These findings demonstrate a strong predictive link between oocyte quality and early embryo viability, providing embryologists with earlier, more informed decision points.
Why This Matters
Being able to predict embryo development from a single egg image could transform treatment planning and resource allocation in IVF.
OQ scoring may help:
- Refine fertilisation and culture strategies
- Prioritise oocytes in cycles with limited embryo creation
- Inform patient counselling with more accurate expectations
- Improve outcomes in egg donation or regulated treatment environments
A Step Toward Earlier, Smarter IVF Decisions
This study suggests that the earliest stages of embryo development can be anticipated before fertilisation even occurs.
By applying AI to oocyte images, we may gain critical foresight into which gametes are most likely to succeed — helping labs work more efficiently and patients feel more informed from the very beginning.
Limitations and Future Directions
Limitations
- As a retrospective study, causal relationships cannot be confirmed
- CHLOE models are trained on blastulation outcomes, which may bias predictions
- Findings are based on data from a single clinic
Next Steps
- Validate the model across more diverse clinics and populations
- Test whether OQ scores remain predictive for frozen/warmed oocytes
- Explore combinations with other predictive tools (e.g., sperm quality, uterine receptivity)
Learn More
Visit Avenues to explore more about AI, predictive fertility care, and how we’re changing IVF from the inside out.
View more of our research presented at ESHRE
Learn more about CHLOE OQ by Fairtility